Form 8-K CODEXIS INC For: Mar 12
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_________________________________
FORM 8-K
_________________________________
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
Date of report (Date of earliest event reported): March 12, 2018
_________________________________
Codexis, Inc.
(Exact name of Registrant as Specified in its Charter)
_________________________________
Delaware | 001-34705 | 71-0872999 | ||
(State or other jurisdiction of incorporation) | (Commission File Number) | (I.R.S. Employer Identification No.) | ||
200 Penobscot Drive
Redwood City, CA 94063
(Address of Principal Executive Offices) (Zip Code)
(650) 421-8100
(Registrant's telephone number, including area code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
_________________________________
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
¨ | Written communication pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ¨
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Item 7.01 | Regulation FD Disclosure. |
On March 12, 2018, Codexis, Inc. (the “Company”) updated its corporate presentation (the “Corporate Presentation”) in connection with upcoming investor conferences. A copy of the Corporate Presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K, and incorporated by reference herein.
The information furnished in this Current Report on Form 8-K pursuant to Item 7.01 (including Exhibit 99.1) shall not be deemed to be “filed” under the Securities Exchange Act of 1934, as amended, nor shall it be incorporated into any future filing under the Securities Act of 1933, as amended, or under the Securities Exchange Act of 1934, as amended, unless the Company expressly sets forth in such future filing that such information is to be considered “filed” or incorporated by reference therein.
Item 9.01 | Financial Statements and Exhibits. |
(d) | Exhibits. |
Exhibit No. | Description | |
99.1 | Corporate presentation of Codexis, Inc. | |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
Date: March 12, 2018 | CODEXIS, INC. By: /s/ Gordon Sangster Name: Gordon Sangster Title: Senior Vice President and Chief Financial Officer | |
EXHIBIT INDEX
Exhibit No. | Exhibit Description | |
99.1 | ||

March 2018 Codexis Corporate Presentation
Nasdaq: CDXS
TM

Forward-Looking Statements
• These slides and the accompanying oral presentation contain forward-looking statements that involve risks and uncertainties. These statements relate to
future events or our future financial or operational performance and involve known and unknown risks, uncertainties and other factors that could cause
our actual results, levels of activity, performance or achievement to differ materially from those expressed or implied by these forward-looking
statements. Forward-looking statements include all statements that are not historical facts. In some cases, you can identify forward-looking statements
by terms such as “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “predicts,” “potential” or
the negative of these terms, and similar expressions and comparable terminology intended to identify forward-looking statements. These forward-
looking statements represent our estimates and assumptions only as of the date hereof, and, except as required by law, we undertake no obligation to
update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise.
• Other factors that could materially affect actual results, levels of activity, performance or achievement can be found in Codexis’ Form 10-K for the period
ended December 31, 2016 filed with the SEC on March 9, 2017 and Form 10-Qs filed with the SEC on May 9, 2017, August 9, 2017 and November 9,
2017, including under the caption “Risk Factors.” If any of these risks or uncertainties materialize, or if our underlying assumptions prove to be incorrect,
actual results, levels of activity, performance or achievement may vary significantly from what we projected.
• Our logo, “Codexis,” “CodeEvolver®,” and other trademarks or service marks of Codexis, Inc. appearing in this presentation are the property of Codexis,
Inc. This presentation contains additional trade names, trademarks and service marks of other companies. We do not intend our use or display of other
companies’ trade names, trademarks or service marks to imply relationships with, or endorsement or sponsorship of us by, these other companies.
2

Employees
~120 (70 in R&D)
Company History
16+ Years
Cumulative Investments
> $500m
2018 Revenues (Forecast)
Total = $60-63 million
5Yr CAGR: 13-15%
Patents & Applications
> 1100 worldwide
Codexis – Unlocking the Power of ProteinsTM
3

4
Proteins – Infinite Source of New Value Creating Materials
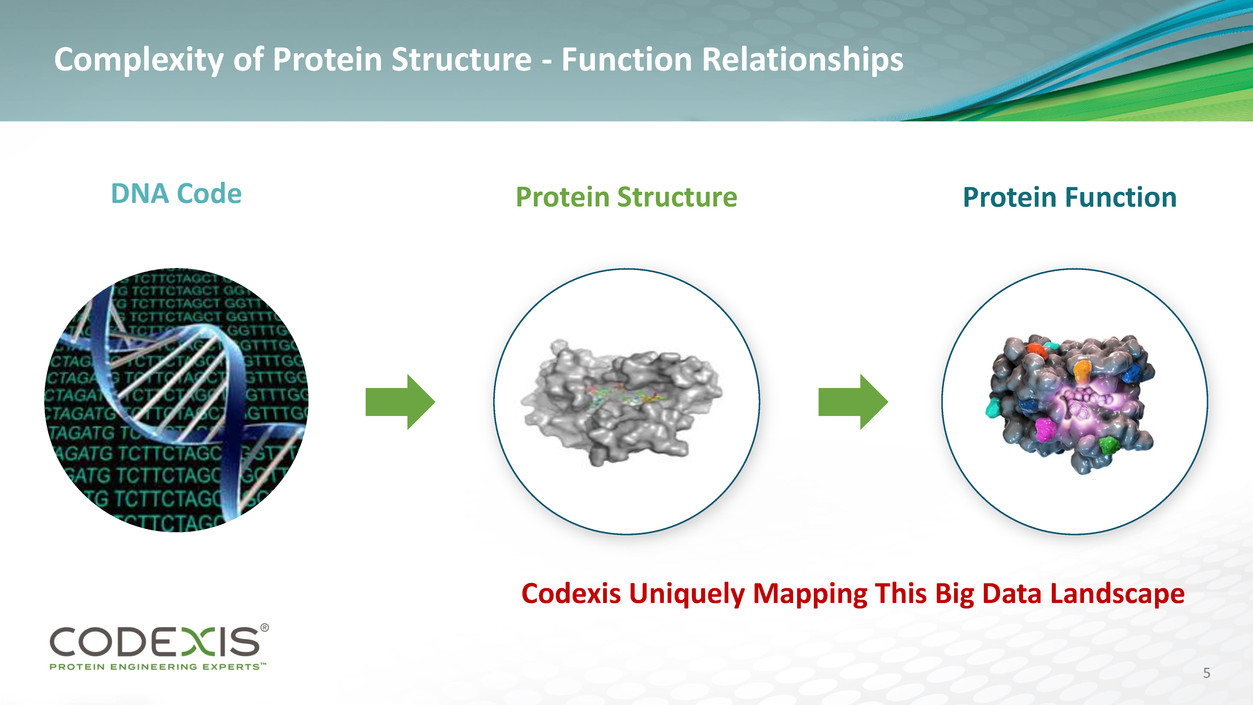
Complexity of Protein Structure - Function Relationships
DNA Code Protein Function Protein Structure
Codexis Uniquely Mapping This Big Data Landscape
5
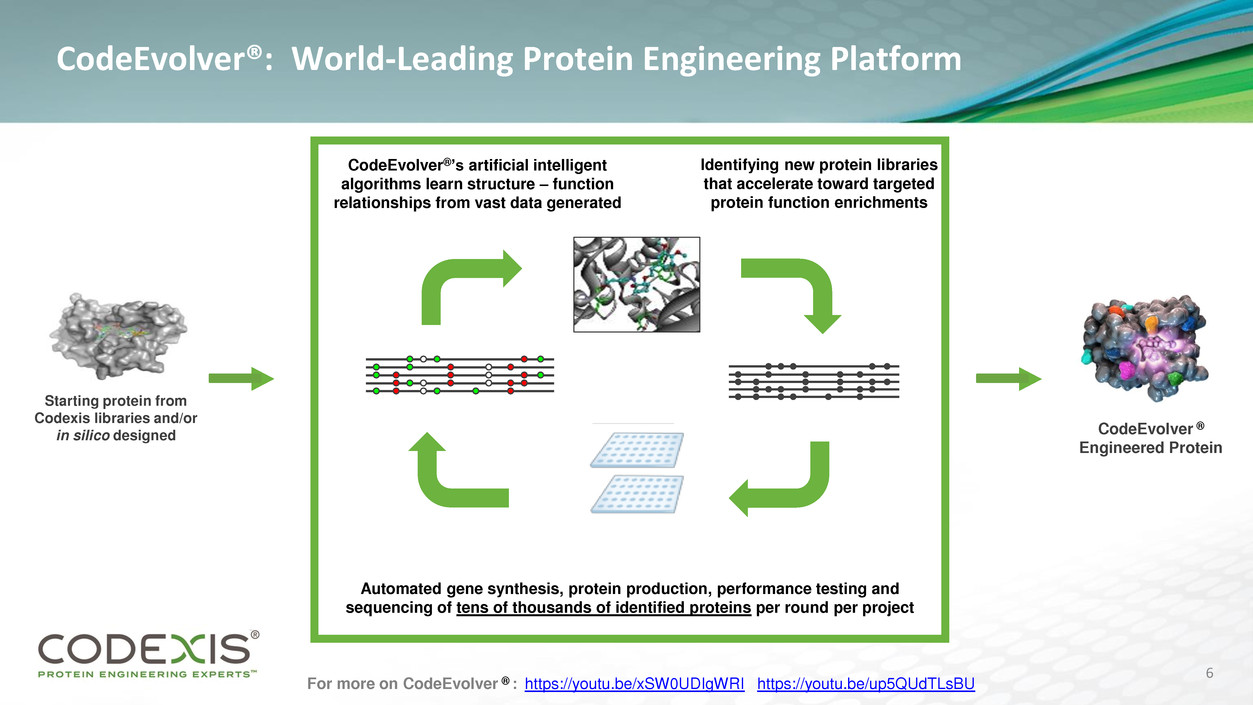
CodeEvolver®: World-Leading Protein Engineering Platform
Starting protein from
Codexis libraries and/or
in silico designed
Identifying new protein libraries
that accelerate toward targeted
protein function enrichments
Automated gene synthesis, protein production, performance testing and
sequencing of tens of thousands of identified proteins per round per project
CodeEvolver®’s artificial intelligent
algorithms learn structure – function
relationships from vast data generated
CodeEvolver ®
Engineered Protein
For more on CodeEvolver ® : https://youtu.be/xSW0UDIgWRI https://youtu.be/up5QUdTLsBU
6
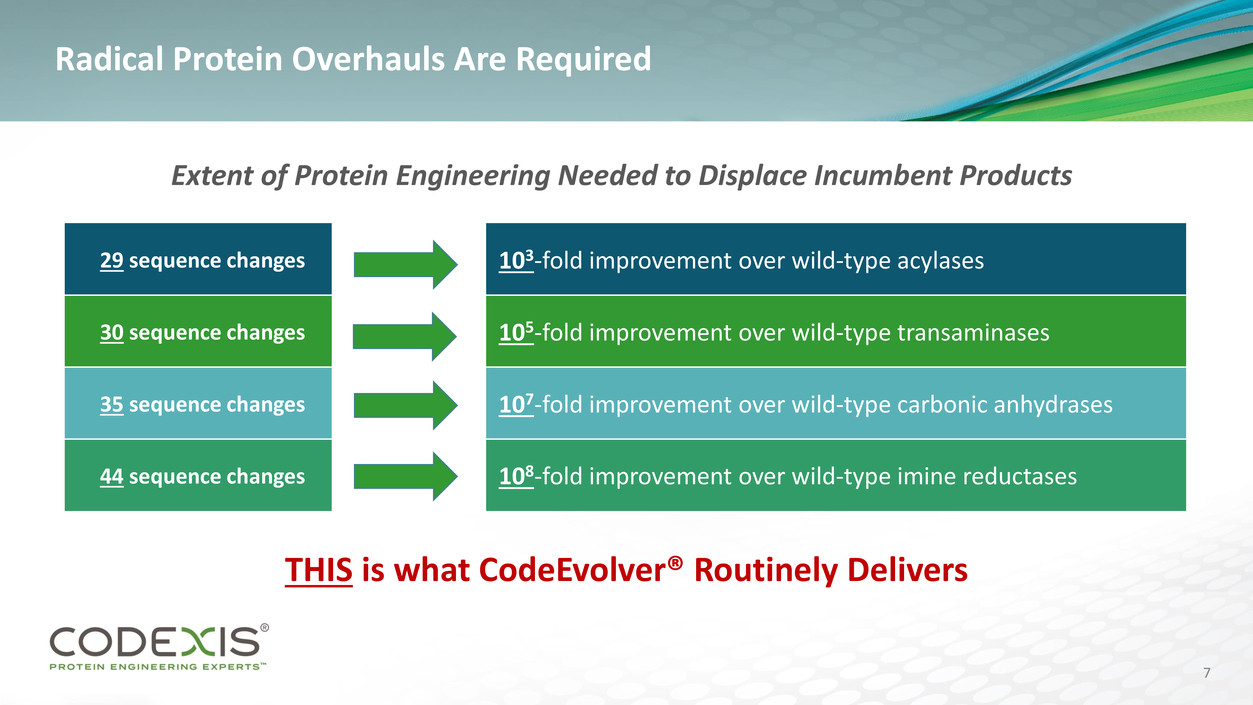
Radical Protein Overhauls Are Required
THIS is what CodeEvolver® Routinely Delivers
Extent of Protein Engineering Needed to Displace Incumbent Products
29 sequence changes 103-fold improvement over wild-type acylases
30 sequence changes 105-fold improvement over wild-type transaminases
35 sequence changes 107-fold improvement over wild-type carbonic anhydrases
44 sequence changes 108-fold improvement over wild-type imine reductases
7
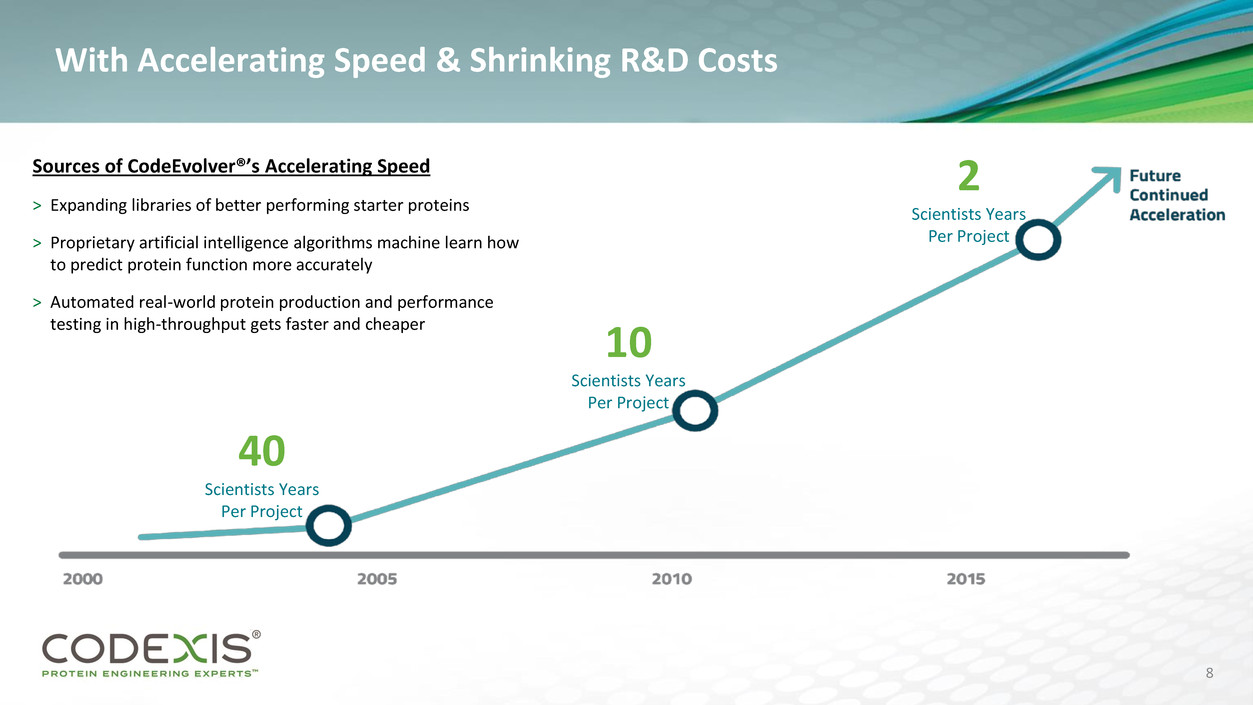
With Accelerating Speed & Shrinking R&D Costs
40
Scientists Years
Per Project
10
Scientists Years
Per Project
2
Scientists Years
Per Project
Sources of CodeEvolver®’s Accelerating Speed
> Expanding libraries of better performing starter proteins
> Proprietary artificial intelligence algorithms machine learn how
to predict protein function more accurately
> Automated real-world protein production and performance
testing in high-throughput gets faster and cheaper
8

9
Accelerating Protein Targets Leveraging CodeEvolver®
Novel, High Performing Industrial Enzymes
Biotherapeutics
Protein Catalysts for Pharma Manufacturing
History: First non-pharma project 2014
Status: Food 10+% of 2017 sales Diagnostics …
TAM: $4+ bn2 industrial enzymes
Comps: Novozymes, Dupont, DSM, Amano
History: 1st target starts 2014; expanded 2017
Status: Pipeline six deep; lead in phase 1 in 2018
TAM: $5+ bn3 enzyme therapeutics
Comps: Biomarin, Shire, Sanofi-Genzyme, Ultragenyx
History: Codexis making the market for 16+ yrs
Status: Accelerating and deepening penetration
TAM: $1+ bn1 can improve > ⅓ of all small molecule drugs
Comps: Limited direct; some in-house big pharma R&D
1) Codexis estimates
2) Markets and Markets Report FB2277, Oct 2016
3) Mordor Intelligence, https://goo.gl/3867rV, Sep 2016

10
Codexis Protein Businesses Leveraging CodeEvolver®
Solid, Accelerating
Financial Base
Protein Catalysts for Pharma Manufacturing

Codexis: A Leader in Pharma Protein Catalysts
Hazardous
Chemicals
Waste
Protein Catalyst Processes
• Lower impurities
• Higher yields
• Fewer process steps
• Less waste
• Energy efficient
Traditional Chemical Process
Hi Pressure
Reactors
Minimal
Waste
Codexis Protein Catalyst Process
Lo Pressure
CSTR
Engineered
Protein
Catalyst
ZERO activity from all starter protein sources
> 10%: higher yield / lower waste & energy
> 50% higher volumetric productivity
Stable in high temp + high organic solvent load
Expensive, toxic chemo-catalyst replaced
No new factory needed to support growth
Merck’s Januvia®
Codexis’ Award Winning Pharma Process Commercialization
“ …[Codexis] helped avoid the cost of
building a 2nd factory to meet the rising
demand for Januvia®.”
Skip Volante, Merck VP R&D
11

Protein Catalyst Pharma Penetration Metrics 2016 2017
2018
(forecast)
Product Sales ($m/yr) 1 ~ $15 ~ $24m
+
Gross Margin on Product Sales (%) 2 36% 46%
# Pharma Customer’s Products Using > $500k of Product 1 4 12
# API’s Commercially Using Codexis 3 7 7
Pre-commercial (phase 2 or later) Pipeline Projects 3 10 15
# Pharma Customers w/ Dedicated Protein Engineer Teams 1 3
# Pharma Customers w/ CodeEvolver® Platform License 2 2
# CodeEvolver® Licensees Generating > $1m Backends 0 0 1
Pharma Protein Catalyst Business: Solid, Accelerating Financial Base
1) Excludes product sales into food application
2) Gross margin on all product sales (includes sales into food)
3) See pipeline snapshot appendix; first three pharmaceutical manufacturing rows only
Platform deals’ 100% margin backends kick in
Deeper R&D access deals with elite customers
Momentum of Codexis catalyst installations
Acceleration of core P&L measures
12

13
Codexis Protein Businesses Leveraging CodeEvolver®
Novel, High Performing Industrial Enzymes
Protein Catalysts for Pharma Manufacturing
History: First non-pharma catalyst project 2013
Status: Food 10+% of 2017 sales Diagnostics …
TAM: $4+ bn 2 industrial enzymes
Comps: Novozymes, Dupont, DSM, Amano
Faster Commercializing,
Larger Protein Targets
Solid, Accelerating
Financial Base

~ $5 Billion
Industrial Enzyme
Sectors
Chemicals & Energy Animal Feed & Health Flavors and Fragrances
Detergents Molecular Diagnostics & Biology
Food and Nutrition
Bring CodeEvolver® Improved Enzymes To Large Existing Markets
14

Codexis Novel Enzymes Enabling
Enhanced Molecular Diagnostics & Biology
Fast Enzyme Commercializations in New Industrial Verticals
2016: Identified opportunity to bring CodeEvolver®
engineered enzymes for the ~ $100m genomic
diagnostic workflow market
2017: First enzyme, a DNA Ligase, engineered and
scaled. Demonstrates 90+% conversions in 3min
vs competitive enzyme at < 50% conversion in >
10 min
2018: DNA Ligase set for market penetration and sales
2018: Second, in a stream of planned enzymes being
engineered and prepped for beta testing
Expect another significant new deal in another
industrial enzyme vertical to be executed in 2018
Codexis Novel Enzymes Enable
Tate & Lyle’s Food Ingredient Innovations
2014: 7 months of CodeEvolver® protein engineering
drove 10-fold reduction in catalyst cost
2015: Lower cost enables Tate & Lyle to launch its
new food ingredient
2017: Sales to T&L for this application are Codexis
2nd largest product sale
2017: Codexis and Tate & Lyle strike second deal to
enable a second, larger new food ingredient
2018: 2nd project’s enzymes scaling for GRAS
affirmations and T&L commercial scale trials
> 10% of Codexis sales to food industry in 2017
15

16
Proving our Capabilities to
Monetize the World’s Highest
Value Proteins
Novel, High Performing Industrial Enzymes
Biotherapeutics
Protein Catalysts for Pharma Manufacturing
Faster Commercializing,
Larger Protein Targets
Solid, Accelerating
Financial Base
Codexis Protein Businesses Leveraging CodeEvolver®

The Opportunity for CodeEvolver® in Biotherapeutics
Stability
Enhanced half-life in plasma and
lysosome, stable in GI-tract
Safety Reduced immunogenicity
Manufacturability
Optimized biophysical properties
for manufacturing
Convenience
Reduced dosing frequency, more
desirable delivery (ie, oral)
Efficacy Enhanced, tissue-specific activity
CodeEvolver ® Targetable BioTx Characteristics
Enzyme deficiencies: 147
Known
prevalence
655
Bibliographic
data 2,019
Known rare
diseases
~7,000
Substantial Rare Disease Enzyme Targets*
* http://sid.usal.es/idocs/F8/FDO26770/Prevalencia_ER_lista.pdf 17

Oral Enzyme For PKU Successfully Developed & Partnered
Nestlé & Codexis Therapeutic Development Platform Access Partnership Deal (Oct 2017):
Purchased rights to commercialize the oral PKU drug candidate
Up to $357 million in upfront + milestones, plus up to low double digit % royalty on sales
First look rights for Nestlé for other IEAAM programs in Codexis pipeline
CodeEvolver® R&D capacity newly dedicated to novel breakthrough protein targets
“We have partnered with Codexis to accelerate enzyme
innovation for multiple health conditions.”
—Nestlé Health Science
18

Codexis phase 1
trials in 2018
NHSc option
early 2019
Setting Up For More Success From Pipeline
CodeEvolver® Generated in vitro
Candidate Discovery
Preclinical Research IND Enabling Human Trials
19
CDX-6114: Inborn Error of Amino Acid Metabolism Disease, PKU
IEAAM - 3
LSD-2
DT4 Discovery Partnership
Drug Target 1 (DT1) Lysosomal Storage Disease LSD-1
IEAAM - 2
Two Add’l Partnerable Assets in 2019
+ Prospects of other significant new discovery partnering
deals working CodeEvolver® into new biotherapeutic
modalities (outside enzyme therapeutics)

Codexis in 2017: Building Momentum Again
20
2017 Product Sales
+74% vs 2016
$26.7m
2017 Total Revenue
Growth Despite $22.5m in
Non-recurring 2016 Revenues
$50.0m
Performance vs Annual Guidance
Fourth Consecutive Year
2017 Product Margin
Up From 36% in 2016
46%
13 Customer Products Each Use > $500k Codexis Protein Catalysts
Food Industry Generates Greater Than 10% of Revenues
Three Pharma Customers Secure Dedicated Teams (up from one)
Breakout New Partnering Deals Executed:
• Biotherapeutics Partnership with Nestlé Health Science
• Second Major Food Ingredient Partnership with Tate & Lyle
MDx/NGS Enzymes Setup For Successful Market Entry
Capably Building Biotherapeutic Development Capabilities:
• Accelerated CDX-6114 for PKU Towards Phase 1 Trials
• Four More Discovery Programs Move Towards Partnerable Status
Completed $25 Million Financing to Support Growth
2017 Strategic Deliverables
Met

21
Codexis 2018 Financial Outlook
$32
$35
$42
$49
$50
$60
$20
$25
$30
$35
$40
$45
$50
$55
$60
$65
$70
2013 2014 2015 2016 2017 2018F
Total Revenue ($ million)
$63
Total Revenues: $60 - 63m (+20-26% vs 2017)
Product Sales: $25 - 28m (2017 = $26.7m)
Product Gross Margin: 45 - 48% (2017 = 46%)
Revenues: ~35% in 1H’18 / ~65% in 2H’18
R&D + GS&A Expenses: Similar To 2017; ~ Smooth Quarterly
Additional Insights Into 2018 Financial Outlook
2018 Annual Guidance Introduced

22
Codexis Strategic Objectives for 2018
Relentless Focus on CodeEvolver® technology platform, AI-Driven Acceleration of Protein Discoveries
Reinforce our powerful, product-commercializing uniqueness in the world’s growing synthetic biology landscape
Continue Profitable Penetration of Protein Catalysis in Pharmaceutical Manufacturing
Continue expansion of the number of our late-stage installations (Phase 2 to commercial) in our pipeline (22 as of 6/30/17)
Deepen deployment by account: Lightly engaged Project Dedicated team CodeEvolver® deal ( 100% margin backends)
At least one significant new deal executed in 2018
Continue to Broaden our Industrial Enzyme Capabilities Outside Pharma Manufacturing
Food: approach commercialization of the second (larger peak rev) project with Tate & Lyle; again > 10% of total revenues
Diagnostics/NGS: penetration / sales established with our DNA Ligase; launch at least one new product into field
At least one significant new deal in another industrial enzyme vertical executed in 2018
Establish the Significance of our Biotherapeutics Business
CDX-6114 For PKU: start phase 1 trial (mid-2018 - $4m cash); Nestlé Health Science option exercise (early 2019 - $3m); advances from there
Pipeline: at least two additional lead candidates (beyond PKU) are “partnerable” (locked and on way to IND) by 2019
At least one significant new deal working CodeEvolver® platform into new biotherapeutic modalities (outside enzyme therapeutics)

Unlock the power
of proteinsTM
23

Contact Us
John Nicols
President & Chief Executive Officer
• [email protected]
• (650) 421-2388
Gordon Sangster
Senior Vice President & Chief Financial Officer
• [email protected]
• (650) 421-8115
Jody Cain
Lippert Heilshorn & Associates
• Codexis Investor Relations Partner
• [email protected]
• (310) 691-7100
Corporate Headquarters
200 Penobscot Drive
Redwood City, CA 94063
USA
Nasdaq: CDXS
24

Appendix – Most Recent Pipeline Snapshot
25

Codexis Pipeline Snapshot
26
Type of Protein & Target Market
Pre-Commercial Commercial
Pipeline Total
6/30/17
vs. prior pipeline
6/30/16
Codexis Driven
Sustaining
Revenues
Codexis
Self-funded
Customer
Partnered
Product Sales and/or
Licensing
Protein Catalysts Improving Pharmaceutical Manufacturing:
Developmental Drugs in Clinical Phase II or later 10 n.a. 10 + 3
Patented On-the-Market Drugs 1 2 3 -
Generic On-the-Market Drugs 1 3 5 9 + 2
Expanding Industrial Enzyme Verticals:
Protein Catalysts For Food Ingredient Manufacturing 3 1 4 -
Enzymes Enabling Molecular Diagnostic & Biology 1 1 -
Novel Biotherapeutics Discovery & Development 5 1 6 + 2
Pipeline Total as of June 30, 2017 7 18 8 33 + 7

Appendix – Success Stories & Customer Testimonials
27

Capital & Yield Efficiencies Unlocked For Merck’s Januvia®
Capacity constraints in existing sitagliptin
(active ingredient in Januvia) supply chain
• Protein engineered from zero activity to
commercial targets in less than 12 months
• 53% higher productivity
• 19% reduction in energy usage
• Expensive, toxic chemo-catalyst replaced
• No new factory needed to support growth
CHALLENGE SOLUTION
“ …[Codexis] helped avoid the cost of building a 2nd factory to meet the rising demand for Januvia®.”
Skip Volante, Merck VP R&D
28

Major PharmaCo Patented Drug Process Overhaul
Inefficient patented drug
manufacturing process
affecting Top-10 global
pharma company’s profit
margins
• Prior protein libraries: zero activity
• 43 sequence variations required
• Engineered protein results in > 3x lower
cost than customer’s target
• Engineered protein’s temperature
stability to fit reaction conditions
• Expensive, toxic chemo-catalyst replaced
CHALLENGE SOLUTION
Aspirational ‘BLUE SKY’ goal
100,000-fold improvement
Minimum target
2700-fold improvement
Engineered Protein Improvements
Phase II+ Drug Processes Overhauled By Codexis Protein Catalysts: 22 & counting
29

CodeEvolver® Licensing Creates Value Across GSK’s Portfolio
“We chose the Codexis platform after a thorough evaluation of the enzyme evolution landscape...”
Doug Fuerst, GSK Technology Development Lead, Synthetic Biology
CHALLENGE SOLUTION
How to effectively leverage protein
engineering widely across
GSK’s portfolio?
• Licensed the CodeEvolver® platform
• Deeply embedded the technology in house
• Applications from discovery to post launch
• Codexis earns front end and back end economics
• Partnership created around shared vision for proteins
and mutual success of CodeEvolver®
30

Healthier food ingredient requires a
lower cost process to enable the
product launch
• Protein catalyst system engineered to meet commercial
targets in less than 7 months
• 20-fold catalyst stability improvement
• 90+% reduction in catalyst system cost
• Enabled commercial production of the healthy
ingredient < 2 years after 1st project discussion
Tate & Lyle’s New Food Ingredient Launch Enabled
CHALLENGE SOLUTION
“We view Codexis as an extension of our internal R&D programs at Tate & Lyle...”
Michael Harrison, Tate & Lyle SVP, New Product Development
31

Create more sensitive, fluid-based
diagnostic tests: earlier, less
invasive cancer detection
• Codexis DNA ligase converts more input DNA to
double-ligated library fragments than T4 DNA ligase
across a range of sample input concentrations
• Exceptional conversion of low concentration (10 ng)
DNA inputs, ideal for liquid biopsy applications
• Codexis DNA ligase achieves maximal substrate
conversion within 5 minutes, enabling streamlined
NGS workflows
Improving Sensitivity and Precision in Molecular Diagnostics
CHALLENGE SOLUTION
Our DNA Ligase is currently being beta tested by selected customers
Other Molecular Diagnostics enzyme candidates are currently being engineered
32

Besides aggressive dietary control,
no available drug treatment
for > 80% of PKU patients
In the USA ~1:15,000
newborns have PKU, causing
lifelong neurocognitive
symptoms
Therapeutic Enzyme to Treat Phenylketonuria (PKU)
CHALLENGE SOLUTION
“Many individuals with PKU are eagerly awaiting new therapeutic options. CDX-6114 holds the
potential of being an attractive treatment for PKU.”
Dr. Gregory Enns, Professor of Pediatrics, Division of Medical Genetics, Stanford University Hospital
• First CodeEvolver® drug discovery breakthrough
• Stability in GI tract enables convenient oral dosage
• > 50-fold stability improvement, in vitro
• Efficacy demonstrated in four preclinical models
• Human trials targeted to start in early 2018
33
Serious News for Serious Traders! Try StreetInsider.com Premium Free!
You May Also Be Interested In
- Codexis to Report First Quarter 2024 Financial Results on May 2
- Skyhigh Security Named a Visionary in the 2024 Gartner® Magic Quadrant™ for Security Service Edge
- Mativ Announces Conference Call to Discuss First Quarter 2024 Results
Create E-mail Alert Related Categories
SEC FilingsSign up for StreetInsider Free!
Receive full access to all new and archived articles, unlimited portfolio tracking, e-mail alerts, custom newswires and RSS feeds - and more!



 Tweet
Tweet Share
Share